Goal of this exercise: Analyze the first study that used the CRISPR Cas9 system to successfully knock out an abnormal gene in human patients (Gillmore et al. 2021). Part 1 provides background information on the disease, method of treatment, and research protocol.
Part 1: Overview of ATTR, CRISPR, and Gillmore et al. 2021
Part 2: Cut the TTR gene with Cas9 under the direction of guide RNA
Part 3: Look for potential off-target effects of Cas9
Part 4: Examine mutations of patients associated with the study
Part 5: Analyze frameshift mutations in monkeys and humans
Organization of Part 1
Overview of Transthyretin Amyloidosis (ATTR).
The CRISPR-Cas9 system for knocking out (disabling) genes.
Overview of study that used CRISPR-Cas9 to knock out the TTR gene.
Note: See the CRISPR exercise to recreate pioneering experiments based on Jinek et al. 2012.
OVERVIEW OF TRANSTHYRETIN AMYLOIDOSIS (ATTR)
Amyloidosis refers to a group of diseases caused by the misfolding of proteins. Transthyretin amyloidosis (ATTR amyloidosis or ATTR) is caused by misfolded transthyretin (TTR), as explained in the video below. Transthyretin is a transport protein that normally carries thyroxine, a thyroid hormone, and also is involved with the transport of vitamin A in the blood serum. One type of ATTR (hereditary ATTR, also called or hATTR or familial ATTR) is caused by mutations in the DNA sequence coding transthyretin. Mutations in the TTR gene result in buildup of misfolded proteins in the nervous and circulatory systems.
A way to treat this condition is to ‘knock out’ (disable) the mutated TTR gene so that it can no longer produce misfolded TTR protein. A study published in July, 2021 gave evidence that the CRISPR-Cas9 system can be used to knock out the TTR gene and reduce serum TTR protein in patients. That study (Gillmore et al. 2021) is the subject of this exercise.
Note: The video below was produced before the Gillmore’s study was published. It refers to ‘gene silencing’ as a potential means to treat ATTR. They are referring to previous attempts to treat ATTR via silencing methods such as RNA interference (RNAi). Gillmore’s study used CRISPR-Cas9 as a gene silencing method. Here is a detailed comparison of these techniques, including advantages and disadvantages of each.
THE CRISPR/CAS9 SYSTEM FOR KNOCKING OUT GENES
Overview: CRISR stands for Clustered Regularly Interspaced Short Palindromic Repeats. It originally evolved as part of the bacterial immune system, to protect bacteria from phage viruses by cutting and disabling viral DNA. Bacteria store viral DNA from previous infections in their genome, then use this information to destroy invading viral DNA by cutting it with CRISPR enzymes.
The most commonly used CRISPR enzyme is Cas9, which stands for ‘CRISPR associated protein 9’. Unlike other restriction enzymes, Cas9 and its relatives can be programmed to cut DNA at a particular location. This is done by creating a unique guide RNA that can recognize and guide Cas9 to that location. The guide RNA incorporates a spacer sequence (usually 20 bases) that will bind to a corresponding sequence on the target DNA via complementary base pairing. Cas9 will then make a double-stranded cut, if a 3-base pair PAM sequence is present close to the cut location. Once the cut has been made, repair mechanisms will join the cut sections back together, but in such a way that the rejoined DNA can no longer be part of a functional gene. Thus the gene has been disabled, or ‘knocked out’.
Discovery: Jinek et al. 2012 was the first study to confirm the function of Cas9 as a programmable restriction enzyme (see the CRISPR exercise to recreate and analyze those experiments). This figure from that publication illustrates how crRNA and tracrRNA bind together to form a crRNA-tracrRNA duplex (‘dual guide RNA’) that brings Cas9 into position and activates it for cutting three base pairs ‘upstream’ of the PAM. The target region and cut site is indicated below, but Cas9 itself is not shown in this figure.
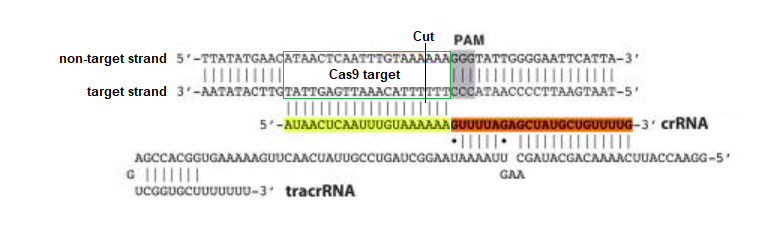
The sequence highlighted in yellow above determines where Cas9 will cut, and can be modified (‘programmed’) to bind to any complementary DNA sequence, unlike other restriction enzymes that can only recognize a single sequence (e.g. EcoRI only recognizes GAATTC). As will be shown, crRNA and tracrRNA can be combined into a ‘single guide RNA’ to make the system more efficient. These Nobel Prize-winning discoveries are of immense practical importance in biotechnology and gene therapy.
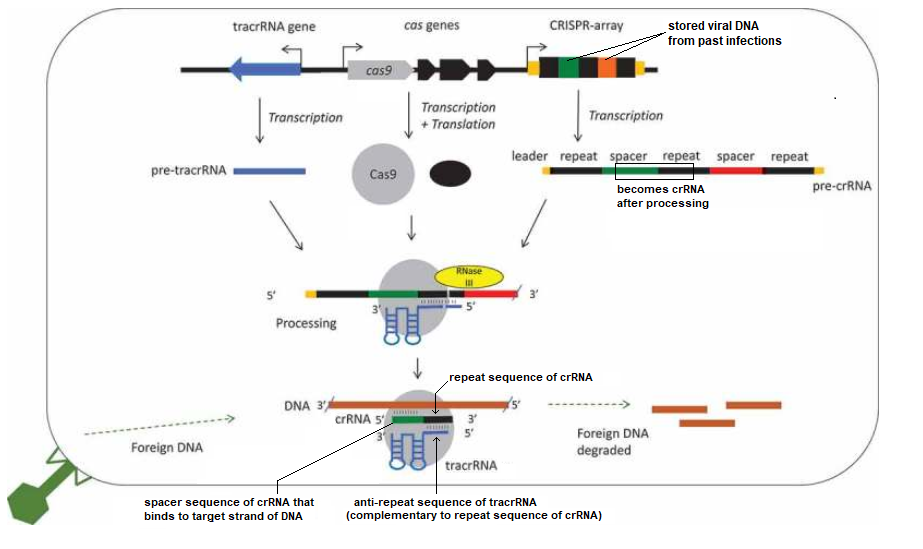
If the above figure represented bacterial DNA, then the orange RNA region would have been transcribed from one of the nearly identical repeat regions of bacterial DNA, and the yellow RNA region would have been transcribed from one of the unique spacers between the repeats. Spacers are sequences of viral DNA that have been stored after previous infections, so that bacteria can recognize them if they are infected again. Together the spacer and repeat region make up ‘crispr RNA’, abbreviated crRNA. There is a separate bacterial gene that encodes for ‘trans-activating crispr RNA (tracrRNA), which associates with the spacer part of crRNA to form guide RNA.
Figure 1 of Chyou and Brown (2018) provides an overview of the CRISPR system in bacteria. Two spacers are shown in this figure, one colored green and the other orange, and the repeats between them are colored black. The legend below the figure explains the process in detail, using the green spacer as an example. The PAM site is not shown, but would be present on the viral DNA close to the Cas9 cut location. Click to enlarge.
Figure legend: “A schematic diagram of a type II CRISPR-Cas9 system focusing on the roles of tracrRNAs in processing and interference. A CRISPR array is shown with repeats in black and spacers in colour, cas genes and the tracrRNA gene are usually nearby. Cotranscribed genes are shown with an arrow. The distances, order and direction of transcription of the genes differ between species, a common arrangement with divergent promoters for cas9 and tracrRNA is shown (see Results). The non-coding RNAs are transcribed into pre-crRNA and pre-tracrRNA, and the proteins transcribed and translated. A 5ʹ leader and 3ʹ trailer is shown for the crRNA (orange)[9]. The RNAs and Cas9 (grey circle) form a complex, specific base pairing occurs between the crRNA repeat (black) and the tracrRNA anti-repeat (blue). Both the crRNAs and tracrRNAs are processed, the crRNA at both ends and tracrRNA at the 5ʹ end. This involves a host factor RNase III (yellow). The complex targets and degrades foreign DNA, though a specific crRNA spacer-DNA protospacer interaction.” —Chyou and Brown 2018
Note: CRISPR researchers often refer to the spacer sequence as the ‘protospacer’, but technically that term refers to DNA from the invading phage virus, before it is stored as a ‘spacer’. CRISPR terminology can be confusing, so be sure to watch the short video linked directly below.
PAM sequence: DNA will not be cut by Cas9 unless a PAM sequence is also present, as explained in this short video. For Cas9, the standard (canonical) PAM sequence is 5′-NGG-3′, where N stands for any of the four DNA bases. The cut is made 3 bp ‘upstream’ from the PAM site (vertical line in Jinek’s figure). Gillmore et al. 2021 used the standard PAM sequence for their ATTR research, but other PAM sequences can be used depending on the circumstances. A list of those sequences can be found here, along with a video explaining PAM function in detail. CRISPR applications are possible because the Cas9-guideRNA complex can scan an entire genome for target sites in a very short period of time.
Guide RNA: ‘Guide RNA’ is a general term that applies to any association of crRNA and tracrRNA involved in the activation of Cas9. In natural systems, crRNA and tracrRNA are transcribed from separate parts of the bacterial genome and then associate via complementary base pairing as shown in the diagrams above. For most CRISPR applications, crRNA and tracrRNA are synthetically linked together to form a ‘single guide RNA’ (sgRNA). This was first done by Jinek et al. 2012 and later refined to make the process more efficient, resulting in a variety of guide RNA designs for specific applications.
In the figure below, crRNA has been linked to tracrRNA by a four-base tetraloop. The crRNA repeat : tracRNA anti-repeat duplex is the region of complementary base pairing between crRNA and tracrRNA (compare with the figure from Chyou and Brown above). When synthetic guide RNA is made, this duplex is shortened, as you can see by comparing the figure below with the figure from Jinek above. Together, this duplex plus the remainder of the tracrRNA (folded into stem loops) functions as a scaffold that binds to and activates Cas9. Thus, you can think of sgRNA as consisting of a spacer (the part that binds to DNA) and a scaffold (the rest of the single guide RNA molecule). This additional figure and legend from Chyou and Brown 2018 describes how the scaffold components interact with Cas9.
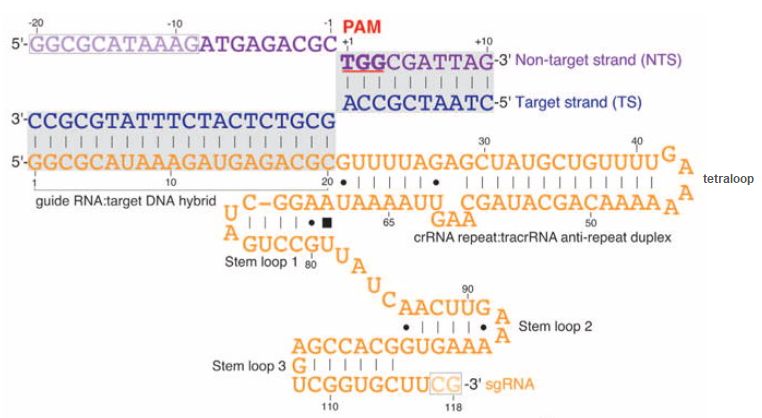
The side of the DNA molecule that binds to guide RNA by complementary base-pairing is called the target strand, and the opposite side is the non-target strand. Note that the 5′-NGG-3′ PAM is part of the non-target strand. The mechanism by which Cas9 cuts DNA involves a temporary displacement of the non-target strand, which is why a portion of the non-target strand is displaced upwards in the above diagram – see Jiang et al. 2016 for details.
The diagrams above are highly schematic. An excellent summary of the CRISPR/Cas9 system can be found here, including three-dimensional depictions of system components.
Considerations for guide RNA design: A major consideration is to avoid ‘off-target effects’ that would occur if Cas9 cut at locations other than the target site, due to the possibility of Cas9 cutting even if there is not a perfect match between the guide RNA and target strand of DNA. Mismatches close to the PAM site interfere with Cas9 activity, but mismatches further away may not prevent Cas9 from cutting DNA.
A variety of strategies have been devised to minimize the changes of off-target effects. For example, the GC content (percentage of the sequence made up of G’s and C’s) should be between 40-80% for increased stability. Some guide RNA sequences can have “hairpins” because of base-pairing within the sequence, which can reduce efficiency. This is called self-complementarity. Various tools for guide design take these and other factors into account when they are used to calculate efficiency scores.
Although not employed by Gillmore et al. 2021, the dual-nickase strategy can be used to minimize off-target effects. This video explains the strategy and provides a detailed example of its use in a knockout experiment. It involves using mutated versions of Cas9, each of which cuts only one strand of the targeted DNA. The video also describes the use of Cas9 mutants requiring a complete match with a full 20 base target site, greatly reducing the chance for off-target effects. See the AddGene CRISPR Guide for more information on increasing enzyme specificity.
OVERVIEW OF GILLMORE ET AL. 2021
A guide RNA-Cas9 system was designed that incorporated the 20 base spacer sequence AAAGGCUGCUGAUGACACCU. This sequence matches a complementary DNA sequence located on the TTR gene of human chromosome 18. The guide RNA sequence was chosen after extensive research to ensure that the system would knock out the TTR gene without causing harmful off-target effects elsewhere in the genome (see Part 3). This is because Cas9 can bind even if mismatches occur between the guide RNA spacer sequence and DNA target strand, as explained in the blue box below.
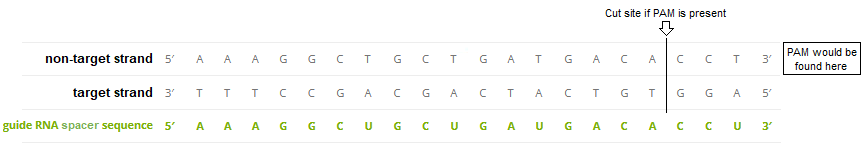
“Cas9 will only cleave a given locus if the gRNA spacer sequence shares sufficient homology with the target DNA. Once the Cas9-gRNA complex binds a putative DNA target, the seed sequence (8-10 bases at the 3′ end of the gRNA targeting sequence will begin to anneal to the target DNA. If the seed and target DNA sequences match, the gRNA [spacer sequence] will continue to anneal to the target DNA in a 3′ to 5′ direction [right to left in the above diagram]. Thus, mismatches between the target sequence in the 3′ seed sequence completely abolish target cleavage, whereas mismatches toward the 5′ end distal to the PAM often still permit target cleavage.” Source: AddGene CRISPR Guide
Note: The 3′ to 5′ direction in the above quote refers to the ends of the guide RNA spacer sequence and the ‘non-target strand’ of DNA, which are identical except for the U’s in the spacer sequence being replaced by T’s in the DNA sequence.
To deliver the CRISPR-Cas9 to patients, Gillmore’s team encapsulated Cas9 messenger RNA (mRNA) and the guide RNA in lipid nanoparticles that were administered intravenously. Once inside liver cells, the guide RNA and Cas9 mRNA was released, and the cell machinery translated the Cas9 mRNA into functional Cas9 enzymes. Guide RNA then transported Cas9 to the TTR gene to make the cut. This therapeutic system is called NTLA-20.
Additional information about the study is given below, as a series of quotes from Gillmore et al 2021. [The article can be obtained in its entirety via free registration at the New England Journal of Medicine.]
Preclinical Studies: “An sgRNA targeting the TTR sequence AAAGGCUGCUGAUGACACCU (human genome build hg38, chromosome 18: 31592987–31593007) was selected for efficient knockout and specificity after a comprehensive off-target characterization workflow that applied a combination of both computational modeling and empirical approaches.”
Methods: “After conducting preclinical in vitro and in vivo studies, we evaluated the safety and pharmacodynamic effects of single escalating doses of NTLA-2001 in six patients with hereditary ATTR amyloidosis with polyneuropathy, three in each of the two initial dose groups (0.1 mg per kilogram and 0.3 mg per kilogram), within an ongoing phase 1 clinical study.”
Patients: “The patients were 46 to 64 years of age, and four of the six patients were men; the body weight ranged from 70 to 90 kg. Three patients had a p.T80A mutation, two a p.S97Y mutation, and one a p.H110D mutation. [Note: Notation such as ‘T80A’ refer to a change in amino acids at a particular location on the gene, as detailed in Part 4 of the exercise.] Three patients had received no previous therapy, and three had previously received diflunisal. All six patients had sensory polyneuropathy in the absence of motor symptoms (polyneuropathy disability score of 1) and a New York Heart Association heart failure class of I”.
Results: “Preclinical studies showed durable knockout of TTR after a single dose. Serial assessments of safety during the first 28 days after infusion in patients revealed few adverse events, and those that did occur were mild in grade. Dose-dependent pharmacodynamic effects were observed. At day 28, the mean reduction from baseline in serum TTR protein concentration was 52% (range, 47 to 56) in the group that received a dose of 0.1 mg per kilogram and was 87% (range, 80 to 96) in the group that received a dose of 0.3 mg per kilogram.”
Conclusions: “In a small group of patients with hereditary ATTR amyloidosis with polyneuropathy, administration of NTLA-2001 was associated with only mild adverse events and led to decreases in serum TTR protein concentrations through targeted knockout of TTR.”
Top of this page
Go to Part 2: Cut the TTR gene with Cas9 under the direction of guide RNA
Go to Part 3: Look for potential off-target effects of Cas9
Go to Part 4: Examine mutations of patients associated with the study
Go to Part 5: Analyze frameshift mutations in monkeys and humans