Goal of this exercise: Analyze experiments by Kariko et al. 2005* that led to the development of mRNA vaccines for COVID and other disease applications. Part 1 provides background information on the human immune system and also describes the research protocol, including cytokine ELISA and flow cytometry.
*Katalin Kariko and Drew Weissman have won over 50 awards for this research, including the 2023 Nobel Prize in Physiology or Medicine and a 2021 Lasker award, known as “America’s Nobel”. Their pioneering mRNA research was rejected at the time by funding agencies and major scientific journals, a lesson for persistence in the face of adversity. The Nobel Prize announcement highlighted Fig 3D of their 2005 paper (see Part 3, step 12 of this exercise).
Part 1: Background information on mRNA vaccines, the immune system, and experimental techniques
Part 2: Determining the inflammatory properties of in vitro-transcribed and naturally occurring mRNA
Part 3: Determining the effect of mRNA on the immune response of dendritic cells
Overview of Part 1
Part 1 provides a brief summary of the innate immune system with links to videos that explain the system in detail, along with the methodology behind the Modena and Pfizer vaccines. Details of the experimental methodology are also provided.
Organization of Part 1
1. mRNA vaccine development.
2. Innate immune system: toll-like receptors and cytokines
3. Glossary of terms used in Kariko et al. 2005
4. Cells used for experimental protocol
5. Experimental technique: cytokine ELISA with video timeline
6. Experimental technique: flow cytometry
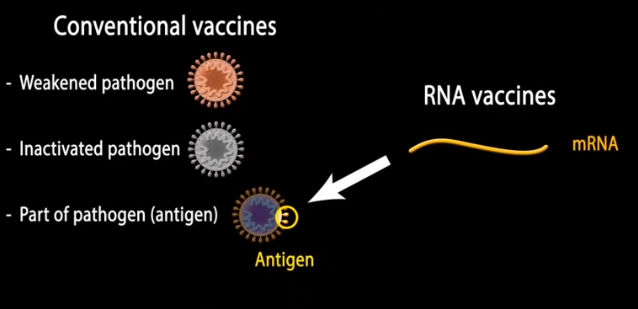
mRNA vaccines: The potential of messenger RNA (mRNA) for vaccine development has long been recognized. If a way could be found to have the body’s own machinery manufacture parts of pathogens, so that antibodies to those pathogens could be created prior to infection, then the immune system could recognize and destroy actual pathogens. For example, using mRNA to manufacture components of the spike protein of the COVID-19 virus would train the immune system to develop antibodies that recognize the spike protein, tagging actual COVID-19 viruses for destruction. Click the image below to see an animation of this process, then click the back button of your browser to return here.
There were two barriers to the development of such a vaccine: (1) how to get the mRNA inside a cell so that the cell’s natural protein-making machinery could be used, and (2) how to prevent the ‘foreign’ mRNA from causing an excessive inflammatory response*. This exercise covers the pioneering research that was the basis for solving the second problem. The importance of this research cannot be overstated, as many lives have been saved as a result. It opened the door to using mRNA for many other therapeutic applications.
*Without modification, the mRNA could not be given at a high enough dose to produce effective immunity against the spike protein due to dangerously high levels of inflammation.
Click the image below to watch a brief video on principles behind the Pfizer and Moderna mRNA vaccines, and watch this section of another video that proves more detail, part of an outstanding series of videos on COVID-19.
Overview of the immune system: The immune system has two major parts: innate and adaptive. The innate immune system is the first line of defense against infection by viruses and other pathogens, whereas the adaptive system is the way that the body “remembers” past infections. An excellent series of video lectures describe this system in detail and is highly recommended, along with the video that summarizes the entire series. Click the image below to go to the section on the role of toll-like receptors (TLRs) in the innate immune response.
Summary of the innate immune response:
Damage by invading bacterial pathogens causes the release of endotoxins that result in the release of inflammatory cytokines. Cytokines are also produced when endotoxins and viral RNA/DNA bind to specific TLRs. Among other effects, cytokines induce the migration of monocytes and neutrophils from the bloodstream to infected tissue, where monocytes develop into macrophages that, along with neutrophils, can destroy pathogens directly by phagocytosis. Macrophages also release cytokines such as TNF-alpha and IL-8 that indirectly induce additional white blood cells to leave the bloodstream and migrate towards the site of infection. Some inflammation helps enhance an adaptive immune response, but too much can cause systemic inflammation that can be dangerous, leading to excessive fever or shock.
TNF-alpha also causes fever via its action on the brain’s hypothalamus, and in addition causes the liver to release C-reactive protein which activates the complement system, another component of the innate immune system. TNF-alpha can also act on the bone marrow to stimulate the production of additional monocytes and neutrophils. TNF-alpha and IFN-alpha are produced in macrophages via a cascade initiated by toll-like receptors that detect foreign proteins, as explained in the video cited earlier. IFN-alpha activates protein kinase-R to disable viral double-stranded RNA (dsRNA). Increased levels of TNF-alpha, IL-8, and IFN-alpha are thus an indication of a positive inflammatory response, which could be detrimental. This is the reason why Kariko et al. 2005 used these cytokines in their experimental procedure. They used cytokine ELISA analysis to determine levels of these substances.
Glossary of terms used in Kariko et al. 2005
| TERM | DESCRIPTION | USE |
|---|---|---|
| TLR3 | Toll-like receptor that recognizes double-stranded RNA | Cells transformed to express TLR3 were used to determine the effect of mRNA on IL8 production |
| TLR7 | Toll-like receptor that recognizes single-stranded RNA | Same as above, for cells transformed to express TLR7 |
| TLR8 | Toll-like receptor that recognizes GU-rich single-stranded RNA | Same as above, for cells transformed to express TLR8 |
| TLR9 | Toll-like receptor that recognizes CpG DNA | Same as above, for cells transformed to express TLR9 |
| MDDCs | Monocyte-derived dendritic cells cultured in vitro for this study | Used to determine the effect of mRNA on TNF-alpha and IL12 production by dendritic cells |
| IFN-alpha | A type I interferon produced by dendritic cells | Used to generate MDDCs in vitro and also measured to determine the effect of mRNA on DC2 dendritic cells |
| TNF-alpha | Cytokine produced primarily by mast cells, with a variety of roles in the immune response including cell signaling | Used to measuring the immune response by MDDCs exposed to mRNA |
| IL-8 | Cytokine that causes neutrophils to migrate towards sites of infection | Used to measure the amount of stimulation of TLR3, 7, 8, and 9 by mRNA |
| IL-12 | Stimulates the growth and function of T cells | Used to measuring the immune response by MDDCs exposed to mRNA |
| lipofectin | Reagent used for the transfection of cells with DNA or RNA | Negative control and tranfection agent |
| poly I:C | Reagent used to stimulate TLR3, to mimic the effect of foreign antigens on this receptor | Positive control |
| RNase | Nuclease that degrades mRNA | Used as control when determining the effect of natural RNA on TNF-alpha production by MDDCs |
| R-848 | Reagent that activates TLR7 and TLR8, to mimic the effect of foreign antigens on these receptors | Positive control |
| pseudouridine - represented by the Greek letter psi | Isomer of the nucleoside uridine, the most common modification found in various types of RNA | Used to determine if replacement of the nucleoside uridine with pseudouridine affects the ability of foreign mRNA to initiate an immune response |
| N6-methyladenosine (m6A) | Nucleoside modification of adenosine found in most eukaryotes and some viruses | Used to determine if replacement of the nucleoside adenosine with methyladenosine affects the ability of foreign mRNA to initiate an immune response |
| 5-methyluridine (m5U) | Another nucleoside modification of uridine, frequently incorporated into transfer RNA | Used to determine if replacement of the nucleoside uridine with methyluridine affects the ability of foreign mRNA to initiate an immune response |
| 2-thiouridine (s2U) | Another nucleoside modification of uridine, frequently incorporated into transfer RNA | Used to determine if replacement of the nucleoside uridine with 2-thiouridine affects the ability of foreign mRNA to initiate an immune response |
| poly(A) tail | String of AMP molecules added to mRNA at the end of transcription for protection against enzymatic degradation | poly(A)-tail-selected mRNA was used to determine its effect on cytokine production by natural mRNAs |
| CD83 and HLA-DR | Markers for mature activated dendritic cells | Used in flow cytometry experiments |
| CpG ODN | Short synthetic single-stranded DNA with CG sequences that mimic the ability of microbial DNA to stimulate TLR9 | Positive control |
Cells used for research: Kariko et al. 2005 used human 293 cells cells and dendritic cells for their experimental protocol. Human 293 cells were transformed to express the toll-like receptors TLR3, TLR7, TLR8, and TLR9 to determine if mRNA induced these receptors to signal the production of IL-8, a cytokine associated with increased immune response. The most common dendritic cells are derived from monocytes, hence the term “monocyte-derived dendritic cells” or MDDCs. They serve as an intermediary between the innate and adaptive parts of the immune system, presenting antigens from pathogenic microbes to T cells. MDDCs used in Kariko et al. 2005 were generated in vitro by exposure to either GM-CSF and IL-4 or by exposure to INF-alpha (called IFN-alpha generated MDDCs). In addition, two types of naturally-occurring dendritic cells were obtained from blood (referred to in the publication as DC1 and DC2). Increased levels of TNF-alpha, IL-8, and IFN-alpha are thus an indication of a positive immune response, which is why Kariko et al. 2005 used these cytokines in their experimental procedure. They used cytokine ELISA analysis (below) to determine the levels of these substances. Note that the development of dendritic cells is complex and considerable research has been done on this topic since Kariko et al. 2005 was published (e.g. Collin and Bigley 2018).
Experimental technique – ELISA: The video below describes the cytokine ELISA technique, used in this example to determine if antigens from a parasite will stimulate cytokine production by macrophages. This is the ‘sandwich ELISA‘ method, whereby capture antibodies adhere to a 96-well plate, antigens attach to the capture antibodies, and a second antibody (detection antibody) adheres to the antigens. An enzyme is added that adheres to the second antibody and reacts with TMB to produce fluorescence that can be measured*. Although the goal was different, the technique is the same as the one used by Kariko et al. 2005. A timeline is given underneath the video.
*The second antibody has biotin covalently attached. The enzyme has avidin attached, which binds to the biotin on the second antibody. Often the enzyme is directly covalently attached to the second antibody, which saves a step.
| TIME | procedure |
|---|---|
| 5:19 | Coat the plate with macrophages |
| 6:01 | Prepare dilution series of parasite lysate using DMEM cell medium |
| 6:35 | Prepare dilution series of LPS (positive control) using DMEM cell medium |
| 6:50 | Add lysate and LPS to selected wells of a 96-well plate and incubate 24 hours |
| 7:50 | Collect supernate from wells and store for use with ELISA |
| 8:23 | Add capture antibodies to a fresh 96 well plate and incubate overnight (so that they adhere to the plate) |
| 9:10 | Wash plate with PBS |
| 9:45 | Add blocking buffer to prevent non-specific binding of capture antibodies, and incubate on a plate shaker |
| 10:18 | Prepare dilution series of cytokine standard, used to generate a standard curve. The example shown is for TNF-alpha. |
| 11:04 | Wash plate |
| 11:20 | Add cytokine standard series and negative control to plate |
| 12:00 | Add samples from the stored plate (step 5), in triplicate, and incubate on a plate shaker |
| 12:40 | Wash plate |
| 12:45 | Prepare detection buffer that includes the detection antibody (with biotin attached), and incubate on a plate shaker |
| 13:02 | Wash plate |
| 15:35 | Add avidin-HRP (horseradish peroxidase with avidin attached), incubate on a plate shaker, and wash three times |
| 14:00 | Prepare fresh TMB substrate by adding TMB A and TMB together, and protect from light |
| 14:15 | Add TMB to all wells of the plate and incubate in the dark in foil |
| 14:35 | Remove foil and observe color resulting from the oxidation of TMB and the reduction of HRP |
| 14:59 | Add stop solution and place plate in ELISA reader to measure fluorescence |
Experimental technique – Flow Cytometry: The video below describes another experimental procedure used by Kariko et al. 2005. They used flow cytometry to measure activation of dendritic cells by determining the numbers of these cells expressing CD83 and HLA-DR. Increases in activation of dendritic cells is an indicator of inflammation. Kariko et al. 2005 used flow cytometry to measure the effect of various types and modifications of mRNA on monocyte-derived dendritic cells (MDDCs).
Go to Part 2: Determining the inflammatory properties of in vitro-transcribed and naturally occurring mRNA
Go to Part 3: Determining the effect of mRNA on the immune response of dendritic cells